Neuralink, the neurotechnology company co-founded by Elon Musk, has been a subject of both fascination and skepticism since its inception in 2016. Aiming to revolutionize the interface between the human brain and computers, Neuralink has made significant strides in brain-computer interface (BCI) technologies. With the announcement of its first human trial, excitement in both the scientific community and among the public has surged. This article delves into the details of Neuralink’s first human trial, its implications, and what it could mean for the future of neuroscience and technology.
Background of Neuralink
Neuralink’s mission is to create devices that can be implanted in the human brain to facilitate communication between the brain and external devices, thus helping individuals with neurological conditions or injuries. The technology involves ultra-thin, flexible “threads” made from biocompatible materials, which can read and write neural data. These threads are designed to be less invasive than traditional electrodes, with the potential for long-term implantation.
The goal of Neuralink is not only to assist with medical conditions but also to enhance cognitive functions, potentially leading to groundbreaking advancements in human capability.
Objectives of the Human Trial
The inaugural human trial aims to investigate the safety and efficacy of the Neuralink device when implanted in individuals with specific neurological conditions. The primary objectives include:
- Safety Assessment: To evaluate the safety of the device, including any potential risks associated with the implantation process and the functioning of the device.
- Efficacy Measurement: To determine how well the device can interpret brain signals and translate them into actionable commands for computers or other technology.
- Long-Term Effects: To monitor patients over time for any long-term side effects or issues related to the device.
Trial Details
Participants
The trial is expected to involve a small number of participants with severe neurological conditions, such as spinal cord injuries, amyotrophic lateral sclerosis (ALS), or other disorders that impair motor functions. The selection criteria will ensure that only those who could potentially benefit from the technology are considered for the trial.
Procedure
The implantation procedure is minimally invasive, utilizing a specialized robot designed by Neuralink to insert the threads with precision. This robotic system has been tested extensively in animal studies and has shown promising results in terms of accuracy and safety.
- Preoperative Evaluation: Participants will undergo thorough assessments, including medical history and neurological evaluations.
- Surgical Procedure: Under general anesthesia, the robot will precisely implant the threads into targeted areas of the brain.
- Postoperative Monitoring: Participants will be monitored closely following the surgery to ensure recovery and assess the initial functioning of the device.
Duration
The trial is planned to span several months to years, allowing for adequate observation of participants to gather significant data on safety and efficacy.
Potential Impact
If successful, Neuralink’s human trial could have profound implications:
- Medical Advancements: The ability to restore movement or improve communication capabilities for individuals with disabilities is a primary goal, which could significantly enhance their quality of life.
- Neuroscience Understanding: Insights gained from interpreting brain signals could lead to deeper understandings of various neurological conditions.
- Future Technological Integration: Success in this trial could pave the way for broader applications, potentially leading to BCIs that enhance human cognition or memory.
Neuralink’s first human trial, involving a brain-computer interface (BCI) implant, began in January 2024, with the first patient, Noland Arbaugh, successfully able to control a computer cursor with his thoughts.
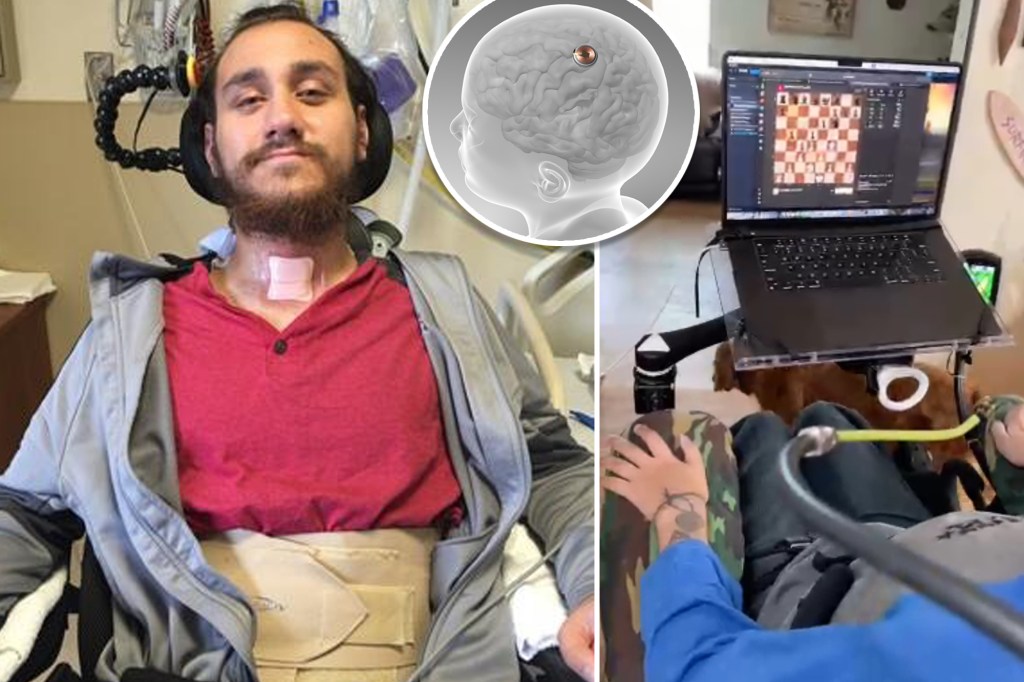
Ethical Considerations
The trial also raises important ethical questions regarding the implications of brain-computer interfaces. Concerns about privacy, consent, and the potential for technology misuse are critical discussions that need to accompany the deployment of such technologies. Ensuring the protection of participants’ rights and data will be paramount.
Conclusion
Neuralink’s first human trial marks a significant milestone in the journey toward integrating technology with the human brain. As researchers and participants embark on this novel venture, the world watches with anticipation. The outcomes of this trial could reshape not only medical practices but also the very concept of human-computer interaction, ushering in a new era of neurotechnology. As we await further developments, the possibilities remain as vast as the human mind itself.
#Neuralink #BrainComputerInterface #Neurotechnology #HumanTrial #ElonMusk #Neuroscience #MedicalInnovation #CognitiveEnhancement #EthicsInTech #FutureOfMedicine #Neuroprosthetics #TechForGood #BrainHealth #Neuroengineering #AIIntegration










Leave a comment